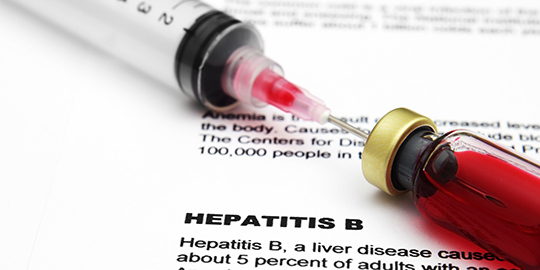
Indian pharma company gets nod to sell hepatitis B medicine in US
United States drug regulator has granted its nod to India’s Aurobindo Pharma to manufacture and sell in the American market the generic drug meant for those infected with hepatitis B virus. Aurobindo Pharma Limited “has received the final approval from the US Food and Drug Administration (USFDA) to manufacture and market Entecavir Tablets, 0.5mg and 1mg,” the company said in a statement on Thursday. The Hyderabad-based firm had sought nod under the Abbreviated New Drug Application (ANDA) which is applicable for generic drugs that are similar to innovator’s medicine in terms of impact on patients. “The approved ANDA is bioequivalent and therapeutically equivalent to the reference listed drug product (RLD) Baraclude® Tablets, 0.5mg and 1mg, of Bristol-Myers Squibb,” the company said, adding, “Entecavir Tablets are indicated for treatment of chronic hepatitis B virus infection of the liver.” The current market size of the product is estimated to be worth $294 million, according to IMS—a global body which keeps track on healthcare industry. Aurobindo Pharma Limited, which manufactures generic pharmaceuticals and active pharmaceutical ingredients, has 209 ANDA approvals from the US regulator so far. The company already has presence in more than 125 countries with its robust product portfolio spread over six major categories including antibiotics, anti-retrovirals, gastroenterologicals, and anti-allergics.
August 27, 2015 | 3:03pm IST.






 to success.
to success.