The new government’s plans to identify new markets and promote Ayurvedic medicines globally, but can it address the real problem of regulatory bottlenecks in the West?
Sachin Manawaria | @TheDollarBiz
 Ayurveda is perhaps the oldest form of medical treatment and is getting popular in both India and overseas due to its holistic approach, low cost and lack of side effects. However, making it a major export product remains a challenge for India, particularly in entering markets such as USA.
The Modi government has announced several measures to promote Ayurveda and Ayurvedic medicines as a part of its national preventive health care policy. It has allocated Rs.5,000 crore to the Department of Ayurveda, Yoga & Naturopathy, Unani, Siddha & Homeopathy (AYUSH), up significantly compared to the Rs.1,200 crore by the previous government.
Important goals set at the recently held World Ayurveda Congress (WAC) in Delhi include: facilitating acceptance and recognition for Ayurveda in all countries, particularly in North America, EU, ASEAN, and UAE; garner the support of the scientific community for Ayurveda; and initiate global research on Ayurveda.
The market for Ayurvedic products is growing as well. As per the estimates of National Medicinal Plant Board, with the global resurgence in traditional and alternative health care, the global herbal trade now stands at around $120 billion which could surge to $7 trillion by 2050.
Ayurveda is perhaps the oldest form of medical treatment and is getting popular in both India and overseas due to its holistic approach, low cost and lack of side effects. However, making it a major export product remains a challenge for India, particularly in entering markets such as USA.
The Modi government has announced several measures to promote Ayurveda and Ayurvedic medicines as a part of its national preventive health care policy. It has allocated Rs.5,000 crore to the Department of Ayurveda, Yoga & Naturopathy, Unani, Siddha & Homeopathy (AYUSH), up significantly compared to the Rs.1,200 crore by the previous government.
Important goals set at the recently held World Ayurveda Congress (WAC) in Delhi include: facilitating acceptance and recognition for Ayurveda in all countries, particularly in North America, EU, ASEAN, and UAE; garner the support of the scientific community for Ayurveda; and initiate global research on Ayurveda.
The market for Ayurvedic products is growing as well. As per the estimates of National Medicinal Plant Board, with the global resurgence in traditional and alternative health care, the global herbal trade now stands at around $120 billion which could surge to $7 trillion by 2050.

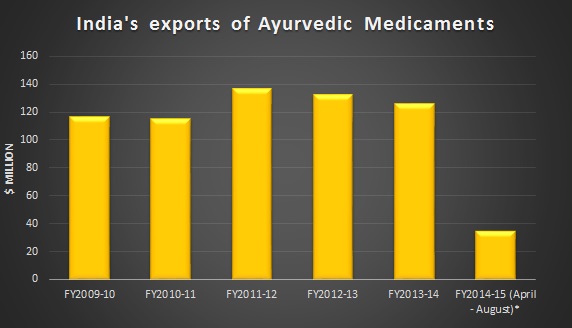
Source - Ministry of Commerce, India
This article was published on November 21, 2014.
 Ayurveda is perhaps the oldest form of medical treatment and is getting popular in both India and overseas due to its holistic approach, low cost and lack of side effects. However, making it a major export product remains a challenge for India, particularly in entering markets such as USA.
The Modi government has announced several measures to promote Ayurveda and Ayurvedic medicines as a part of its national preventive health care policy. It has allocated Rs.5,000 crore to the Department of Ayurveda, Yoga & Naturopathy, Unani, Siddha & Homeopathy (AYUSH), up significantly compared to the Rs.1,200 crore by the previous government.
Important goals set at the recently held World Ayurveda Congress (WAC) in Delhi include: facilitating acceptance and recognition for Ayurveda in all countries, particularly in North America, EU, ASEAN, and UAE; garner the support of the scientific community for Ayurveda; and initiate global research on Ayurveda.
The market for Ayurvedic products is growing as well. As per the estimates of National Medicinal Plant Board, with the global resurgence in traditional and alternative health care, the global herbal trade now stands at around $120 billion which could surge to $7 trillion by 2050.
Ayurveda is perhaps the oldest form of medical treatment and is getting popular in both India and overseas due to its holistic approach, low cost and lack of side effects. However, making it a major export product remains a challenge for India, particularly in entering markets such as USA.
The Modi government has announced several measures to promote Ayurveda and Ayurvedic medicines as a part of its national preventive health care policy. It has allocated Rs.5,000 crore to the Department of Ayurveda, Yoga & Naturopathy, Unani, Siddha & Homeopathy (AYUSH), up significantly compared to the Rs.1,200 crore by the previous government.
Important goals set at the recently held World Ayurveda Congress (WAC) in Delhi include: facilitating acceptance and recognition for Ayurveda in all countries, particularly in North America, EU, ASEAN, and UAE; garner the support of the scientific community for Ayurveda; and initiate global research on Ayurveda.
The market for Ayurvedic products is growing as well. As per the estimates of National Medicinal Plant Board, with the global resurgence in traditional and alternative health care, the global herbal trade now stands at around $120 billion which could surge to $7 trillion by 2050.
 Source - Ministry of Commerce, India
Source - Ministry of Commerce, India





 to success.
to success.